30 Nov Proposal – Scientific Collaboration Italy-Israel 2014
Study and optimization of turbulent mixing processes to enhance microalgae growth for bioethanol production
Scientific Cooperation Italy-Israele, 3rd call
Israel: Tel Aviv University (A.Liberzon),
Italy: Politecnico di Torino (D.Tordella), Università di Catania (M.Gennari).
Abstract
With respect to plant crop derived bio-fuels, algae are currently considered as the most promising alternative renewable source of biomass for the production of bioethanol. The economical sustainability of the microalgae-biofuel production lies in the «small» details of the production, divided roughly into three types of factors: abiotic (light, temperature, nutrients, oxygen, carbon dioxide, salinity, and toxics), biotic (competition, pathogens) and operational (shear-induced mixing, dilution rate, depth of mixing, harvest frequency, etc.). Our proposal, combining the know-how from the micro-algae, turbulence and mixing processes, seeks to improve the operational factors significantly based on our deep understanding of the key mechanism of the turbulent entrainment across stratification layers: 1) air-water interface with its very strong density jump and 2) culture medium layer, with the Lagrangian dynamics of micro-swimmers in a turbulent environment. The aim of this research is to explore the mixing across stratified layers more in-depth and to try to quantify the vertical mixing in a breeding tank for algae bioethanol production (linking biology to hydrodynamics), in view of a potential application at a larger outdoor scale. We will apply theoretical, numerical, and experimental tools to obtain the parameters for the different combinations of the main parameters: Reynolds and Richardson numbers, as well as provide the quantitative output for the two typical installation sites: open/covered ponds/lakes and closed photo-bioreactors (using classical fluid flow manipulators: pipes ejecting air, grids, etc.).
In 2014, the urgent need to address climate change, energy security, and the depletion of fossil fuel reserves drove intensified research into sustainable alternatives to conventional fossil fuels. Among the most promising renewable resources, microalgae emerged as a strong candidate for bioethanol production, owing to their rapid growth rates, high carbohydrate content, and ability to thrive in non-arable environments.
Unlike first-generation biofuels produced from food crops—requiring fertile land and large volumes of freshwater—microalgae were cultivated in seawater or wastewater, avoiding competition with agriculture while reducing environmental impact. They also offered additional benefits, including CO₂ fixation via photosynthesis, wastewater treatment, and higher biomass yields per unit area compared to terrestrial crops. Furthermore, residual algal biomass remaining after oil extraction served as feedstock for multiple fuel types, such as biodiesel, ethanol, and methane.
As part of the BIOALMA project, funded by the Italian Ministry of Environment and Protection of Land and Sea, the Section of Agricultural Chemistry at the University of Catania carried out experiments to optimize growth conditions for high-carbohydrate algal biomass. Promising results were obtained when using farm wastewater, along with optimized illumination and nutrient balance. It was found that nitrogen limitation, combined with sodium bicarbonate addition, significantly increased carbohydrate concentration in the biomass.
However, total biomass production remained limited by insufficient mixing, which caused uneven light exposure and nutrient distribution. This emphasized the necessity for improved tank design with a focus on enhanced suspension mixing. Turbulent mixing was identified as critical for maintaining uniform distribution of light, CO₂, and nutrients, preventing biomass sedimentation, and maximizing photosynthetic efficiency.
In particular, vertical mixing was considered a priority, as it improved CO₂ distribution and created cyclic light exposure for algal cells, leading to enhanced growth. The overall sustainability of microalgae–bioethanol production was recognized as dependent on careful optimization of:
Abiotic factors (light, temperature, nutrients, oxygen, CO₂, salinity, toxins)
Biotic factors (competition, pathogens)
Operational factors (mixing intensity, dilution rate, harvest frequency)
The 2014 project aimed to address operational factors by advancing turbulent entrainment understanding across stratification layers, specifically:
Air–water interfaces with strong density gradients.
Culture medium layers influenced by the Lagrangian dynamics of micro-swimmers in turbulence.
Objectives
The primary objective of the project was the design and development of a fluid handling system to enhance microalgae growth for bioethanol production through an innovative “active turbulence” approach. By increasing momentum and gas exchange across air–liquid and liquid–liquid interfaces in open ponds and photobioreactors, the project sought to improve biomass yield and carbohydrate content.
Research activities included a combination of laboratory experiments and numerical simulations of both sheared and shear-free turbulent mixing.
Specific objectives included:
Characterizing turbulence-driven transport of dissolved gases and nutrients in stratified algal culture media relevant to ethanol production.
Quantifying CO₂ and nutrient dispersion in controlled algae ponds, analyzing mixing efficiency across interfaces through experimental observations, statistical turbulence analysis, and high-resolution simulations.
Identifying optimal flow configurations to maximize the growth of Chlorella vulgaris and Scenedesmus quadricauda in systems utilizing farm wastewater, with the aim of achieving high carbohydrate content as ethanol precursors.
The collaboration with Israeli partners was based on the complementary expertise of the participating research groups in microalgae cultivation, hydrodynamic modeling, and laboratory flow experimentation. This joint effort strengthened scientific collaboration, supported graduate student training, and advanced technological transfer in algae-based biofuel production systems.
METHODOLOGY –
The project combined laboratory experiments, numerical simulations, and biological growth studies to optimize turbulent mixing for microalgae cultivation.
1. Laboratory Experiments – Tel Aviv University
In 2014, the Turbulence Structure Laboratory at Tel Aviv University built a scaled-down experimental setup to replicate the hydrodynamic conditions of bioreactors and open ponds. Controlled turbulence was generated through mechanical agitation (rotating or oscillating grids) combined with gas/liquid injection.
Three experimental techniques were used to investigate mixing across density interfaces (with and without shear):
Particle Image Velocimetry (PIV): Provided spatially resolved turbulent flow statistics at various distances from the interface.
Planar Laser-Induced Fluorescence (PLIF): Quantified algae distribution, degree of mixing, and stratification.
3D Particle Tracking Velocimetry (3D-PTV): Captured the 3D structure of interfaces and the Lagrangian dynamics of algae in response to stratification, turbulence, and shear.
Growth rates were measured under different turbulent mixing strategies and directly compared with numerical simulations (Politecnico di Torino) and biological results (University of Catania) under matched stratification, turbulence, and nutrient conditions.
A PhD student (L. Verso) and an MSc student participated, gaining experience in PIV, PLIF, and 3D-PTV techniques. Collaboration with the PhiloFluid group exposed the students to high-performance computing and direct numerical simulations.
2. Numerical Simulations – Politecnico di Torino (PhiloFluid Group)
The PhiloFluid group carried out high-resolution direct numerical simulations (DNS) of turbulent flow in algae ponds using parallel computing resources such as the IBM BG/Q “Fermi” at Cineca.
The simulations:
Solved the Navier–Stokes equations for stratified flow.
Modeled transport of dissolved gases, nutrients, and microalgae.
Quantified mixing rates, velocity and vorticity field structures, and CO₂ distribution in the culture medium.
These simulations provided 3D concentration fields and parameterizations of stratification levels—data inaccessible in laboratory experiments. A PhD student (L. Gallana) and 1–2 MSc students participated, receiving training in massively parallel computation for CFD.
3. Biological Experiments – University of Catania
The University of Catania conducted growth studies of Chlorella vulgaris and Scenedesmus quadricauda in open systems with farm wastewater under different turbulence regimes.
Culture Conditions:
Medium: BG11 (adjusted to pH 8.4; autoclaved at 121°C for 15 min).
Light: PHILIPS SON-T AGRO 400 lamp (400 W, 55,000 lumens; spectrum optimized for algal photosynthesis) or natural sunlight.
Nutrient Variations: Nitrogen deficiency and bicarbonate addition (NaHCO₃ at 1 g/L and 2 g/L) were tested as CO₂ substitutes.
Experimental Procedure:
Initial growth took place in flasks until the stationary phase; inoculation into growth systems was done at a 5:100 inoculum-to-medium ratio.
Growth was monitored via optical density at 550 nm.
Carbohydrate content was determined by ion chromatography (Dionex DX500, CarboPac PA20 column; acid pretreatment at 30°C for 20 min; quantification of glucose, galactose, xylose via isocratic elution in 20 mM NaOH).
Chlorophyll a and b were measured spectrophotometrically at 665 nm and 649 nm, respectively (UV-vis spectrophotometer Jasco V-530).
Graduate students were actively involved in the experiments. Results were disseminated through seminars to students, faculty, and technical staff at the University of Catania, in collaboration with the Israeli research teams.

Figure 1. Scenedesmus quadricauda (left) and Chlorella vulgaris (right) microalgae are to be used for the production of carbohydrates from which bioethanol is obtained.

Figure 2. Currently used breeding tanks for microalgae (Bioalma project). This apparatus must be improved to increase the mixing of the suspension and thus the production of carbohydrates.
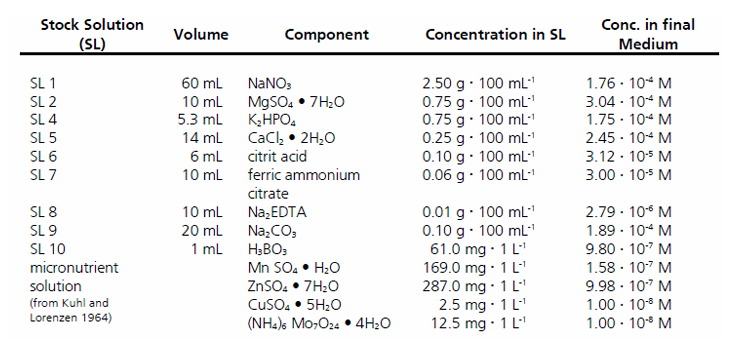
Figure 3. BG-11 substrate composition
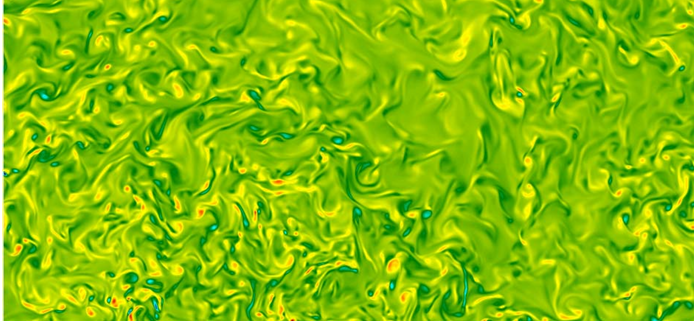
Figure 5. Example of a possible flow obtained by a mixing process, which could be used in a breeding tank. The figure shows the vorticity concentration in a horizontal plane in a turbulent mixing layer (red and blue spots indicate high intensity positive and negative vortices where micro algae could cluster, the green background is the culture medium).

Figure 6. Mixing process: spreading of a horizontal concentration interface by the turbulent fluid motion (dark color indicates higher concentration) as obtained in a direct numerical simulation. The figures show a vertical plane crossing the interface.
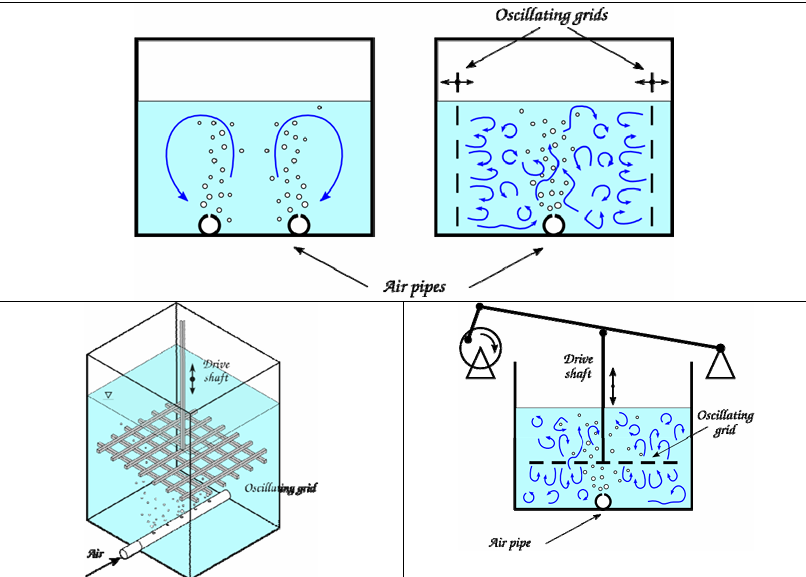
Figure 7. Schemes of possible flow configurations that can enhance the mixing inside the breeding tanks, thus enhancing the dispersion of algae and the supply of air. Top left: multiple air jets issued from holes in the bottom pipes carrying controlled air flows. Top right: enhanced mixing by horizontally oscillating grids. Bottom: mixing enhanced by the addition of a vertically oscillating grid.



